Introduction:
Treatment of therapy-related AML (t-AML) and AML evolving from an antecedent myelodysplastic syndrome (AML-MRC) represent a clinical unmet need being characterized by an unfavorable outcome. Among the emerging treatment options, CPX-351 has been approved by Regulatory Authorities for the treatment of fit patients (pts) affected by these AML sub-types. Although patented for the treatment of pts fit for intensive chemotherapy, in real-life CPX-351 is also administered to unfit ones, raising the case of tolerability in these categories. Among scores for fitness definition, SIE/SIES/GITMO criteria were extensively validated in large cohorts of AML pts receiving intensive chemotherapy and are increasingly incorporated into clinical trials. However, since these criteria have not been tested yet in the subset of t-AML and AML-MRC, we investigated their applicability in these pts receiving CPX-351.
Methods:
Patients classified as t-AML or MRC-AML according to WHO2016 classification receiving at least one cycle of CPX-351 were included in the present analysis. Genetic and cytogenetic data were classified according to ELN2017 risk stratification. SIE/SIES/GITMO criteria were retrospectively applied to the whole series to categorize pts as fit or unfit to intensive chemotherapy (Ferrara et. al, Leukemia 2013), aiming at evaluating early-death rate and overall survival (OS). We also verified if ELN2017 risk-stratification might provide further information about long-term outcome, independently of fitness assessment.
Results:
This retrospective study includes 398 pts with t-AML (19.3%) or AML-MRC (80.7%) enrolled from 29 Italian Institutions between 2018 and 2023. Median age was 65 years (range 32-79), with a slight male prevalence (56.8%).
According to SIE/SIES/GITMO criteria, 323 (81.2%) pts qualified as fit and 75 (18.8%) as unfit. Based on ELN2017 risk stratification, 17 (4.3%) pts were classified as favorable, 162 (40.7%) as intermediate, 217 (54.5%) as adverse risk. Only 2 (0.5%) pts were not classifiable due to incomplete genetic data. No differences were observed in terms of ELN2017 risk distribution among the fit and unfit groups. After first induction, 188 of 323 (58.2%) fit and 42 of 75 (56%) unfit pts achieved a complete remission (CR), for a total of 230 (57.8%) pts entering CR. From CPX-351 start, 18 and 55 deaths at 28 days and at 100 days occurred, respectively. Early death rate at these early timepoints statistically differed between the two groups (3% vs. 10.7% at 28 days and 10% vs. 28% at 100 days for fit and unfit pts respectively; p<0.05). No excess of mortality was observed for unfit patients at any timepoint after 100 days from first CPX-351 induction. Competitive risk analyses showed no differences in terms of early death by relapse between the two groups.
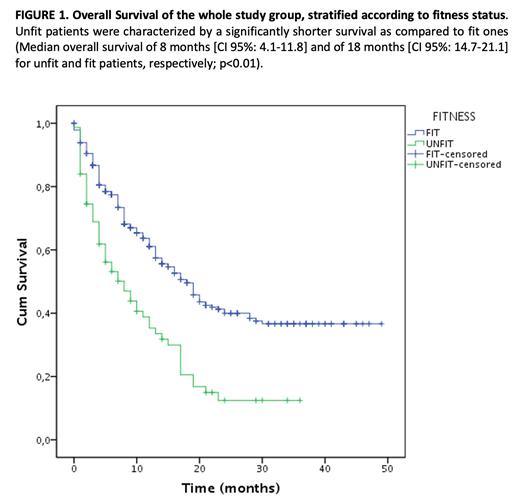
With a median follow up of 24 months, unfit pts were characterized by a significantly shorter OS as compared to fit ones (median OS of 8 months [CI 95%: 4.1-11.8] and of 18 months [CI 95%: 14.7-21.1] for unfit and fit pts, respectively; p<0.0001) [Figure 1]. When stratified according to genetic/cytogenetic, median OS of fit pts was commensurate with ELN2017 risk (median OS not reached vs 20 months vs 13 months for favorable vs intermediate vs adverse risk, respectively; p < 0.01), whereas no differences in median OS were observed between favorable, intermediate, and adverse categories in unfit pts (median OS of 20 months vs 11 months vs 7 months, respectively; p=NS).
Conclusions:
In our real-life analysis, we demonstrated that fitness assessment according to SIE/SIES/GITMO criteria identifies populations with discrete outcome among t-AML and AML-MRC pts receiving CPX-351. In unfit patients, outcome prediction seems independent from ELN 2017 risk stratification. Fit and unfit groups achieved similar CR rates but different long-term OS. Such discrepancy can be justified by the higher early mortality observed among unfit pts that translated into shorter long-term survival. In a future perspective, modulating CPX-351 schedule in this group may be a reasonable option to spare toxicity without giving up the opportunity to deliver curative-intended therapies.
Disclosures
Palmieri:Pfizer: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria. Ferrara:ABBVIE: Honoraria. Martelli:Laboratoires Delbert: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria. Alati:Jazz: Honoraria; AbbVie: Honoraria. Vetro:Jazz Pharmaceuticals: Honoraria; BMS: Honoraria; ABBVIE: Honoraria. Cerrano:Insight Novartis Servier Abbvie Janssen Jazz Astellas Italfarmaco: Honoraria. Mulè:Pfizer: Honoraria; Astellas: Honoraria; Incyte: Honoraria; ABBVIE: Honoraria. Papayannidis:Pfizer, Astellas, Janssen, GSK, Blueprint, Jazz Pharmaceuticals, Abbvie, Novartis, Delbert Laboratoires: Membership on an entity's Board of Directors or advisory committees; Abbvie, Astellas, Servier, Menarini/Stemline, BMS, Pfizer, Amgen, Janssen, Incyte, Novartis: Honoraria. Pagano:AstraZeneca: Honoraria; Moderna: Honoraria; Menarini: Honoraria; Novartis: Honoraria; Pfizer: Honoraria; Gilead: Honoraria; Janseen: Honoraria; Jazz: Honoraria. Buccisano:Abbvie: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Janssen & Cylag: Consultancy, Honoraria; Becton Dickinson: Research Funding; Astellas: Consultancy, Honoraria. Venditti:Janssen: Consultancy, Honoraria, Other: travel support ; Amgen: Consultancy, Honoraria, Other: travel support ; Pfizer: Consultancy, Honoraria, Other: travel support , Speakers Bureau; Jazz: Consultancy, Honoraria, Other: travel support ; AbbVie: Consultancy, Honoraria, Other: travel support ; Medac: Consultancy; Novartis: Consultancy, Honoraria, Other: travel support .

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal